Coronavirus: il test diagnostico per il Covid-19
Come si esegue il test diagnostico per il Coronavirus? Scopriamolo insieme.
Il test per la diagnosi di Coronavirus è un test diagnostico che consiste in un esame di laboratorio che si esegue a partire dalle secrezioni di un individuo per verificare se sia o meno infetto dal Coronavirus.
Quali sono le secrezioni su cui può essere applicato?
In genere si applica sull’ormai famoso tampone faringeo (quindi “in gola”) oppure su altre secrezioni che vengono prese più in basso, dalla trachea o dai bronchi. In questo caso si parla di broncolavaggio. Il test si può applicare in entrambi i campioni perché è lì che si annida il virus: dapprima si replica nella parte alta delle vie aeree, poi passa gradualmente più in basso per arrivare ai polmoni, dove in alcuni limitati casi produce una grande infezione/infiammazione che si chiama polmonite.
Come si svolge?
Il campione viene prelevato dove si trova l’individuo, quindi a casa oppure all’ospedale o dovunque vi sia una persona sospetta. Il prelievo viene svolto con un bastoncino tipo cotton-fioc, messo in una provetta sterile e quindi trasportato fino all’ospedale di riferimento. Non l’ospedale sotto casa vostra, ma un ospedale più attrezzato (ce ne sono uno o due per regione, in genere). I normali laboratori di microbiologia degli ospedali non sono attrezzati per questo tipo di indagini: vengono quindi svolte nei laboratori di microbiologia di secondo livello, che sono appunto identificati dalle regioni e sono in numero limitato.
Come mai il test per il Coronavirus è presente in pochi laboratori specializzati?
Perché le risorse non sono infinite. Molti test specialistici, come quello per la ricerca del Coronavirus, richiedono preparati specifici per essere realizzati.
Un test unico o tanti test simili?
Cerchiamo di capire quale test si utilizza per la diagnosi di Coronavirus tramite test rapido.
Innanzitutto occorre dire che non esiste in tutto il mondo un test univoco che si occupi di diagnosticare il 2019-nCoV. I protocolli attualmente disponibili sono 6, sviluppati da Istituti cinesi, tedeschi, giapponesi, tailandesi e statunitensi. Maggiori informazioni si possono trovare sul sito della World Health Organization (1).
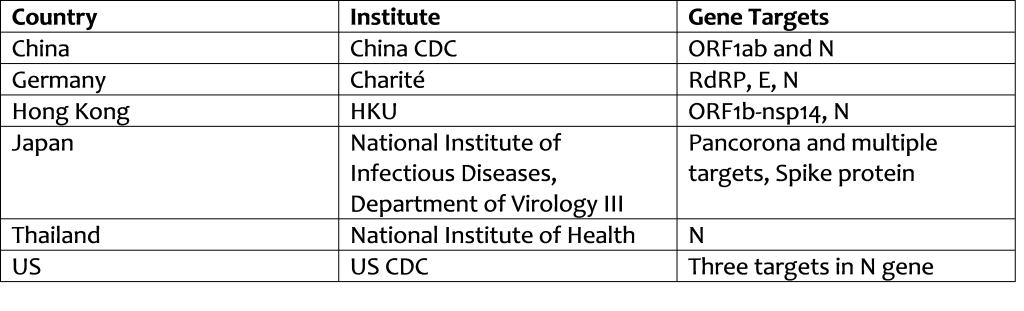
Secondo un protocollo specifico descritto nei dettagli principali che trovate in questo link (2).
Come funziona il test?

Ognuno dei sei distinti protocolli è come se fosse un detective. Va alla ricerca di una specifica impronta del virus. Così come il detective può cercare diverse impronte, anche ciascun protocollo cerca impronte diverse del virus dentro il campione che è stato prelevato dal tampone faringeo o da qualche altra secrezione del paziente da indagare.
Nella figura seguente si può vedere la “impronta” di materiale genetico che va a ricercare il protocollo messo a punto a Berlino.
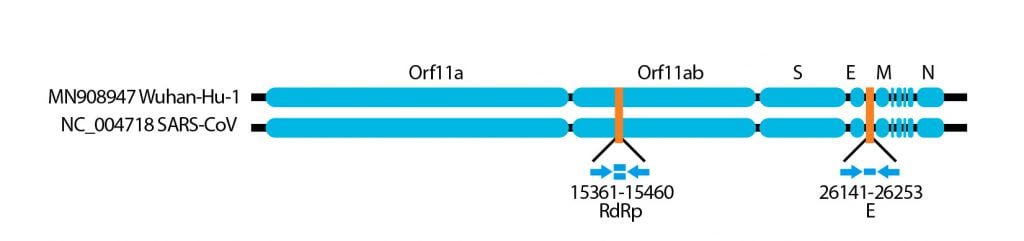
Come funziona tecnicamente? (Se avete già mal di testa saltate questo passaggio e passate all’argomento successivo!)
Per chi vuol saperne di più, diamo qui una delle ricette spiegate in modo semplice:
- Apertura della doppia catena di RNA virale con la giusta temperatura.
- Aggiungere nella miscela dei pezzetti di RNA già pronti, creati dalle ditte specializzate.
- Attivare la RNA polimerasi per la scrittura/amplificazione genica dei pezzi delle impronte virali.
Vari passaggi delle reazioni amplificano il materiale genetico fino a raggiungere milioni e milioni di copie.
Se è presente RNA virale a sufficienza all’interno del campione, esso viene rilevato dal test.
Qui potete trovare un video di spiegazione del sistema PCR che viene utilizzato per compiere questo tipo di indagini: https://www.youtube.com/watch?v=QPOMrRUOMaY
Affidabilità del test
Per essere certi che il test eseguito non faccia “errori” grossolani, come riconoscere altri Coronavirus o altri virus al posto del Coronavirus, sono state condotte delle verifiche specifiche.
Di seguito i risultati ottenuti dopo aver utilizzato il test verificando che non faccia un cross-link per altri Coronavirus.
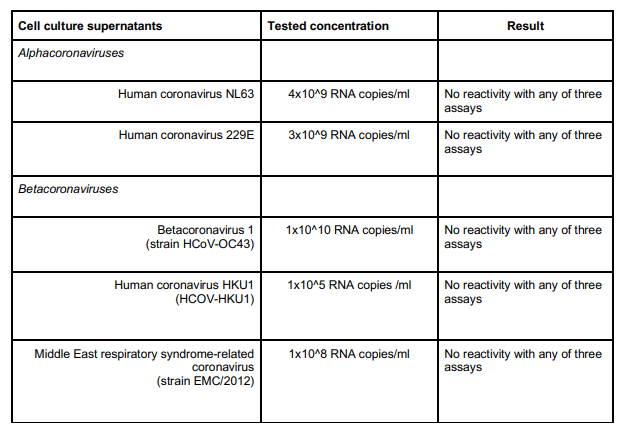
Di seguito la lista degli altri virus respiratori testati, che hanno tutti dato risultato negativo.
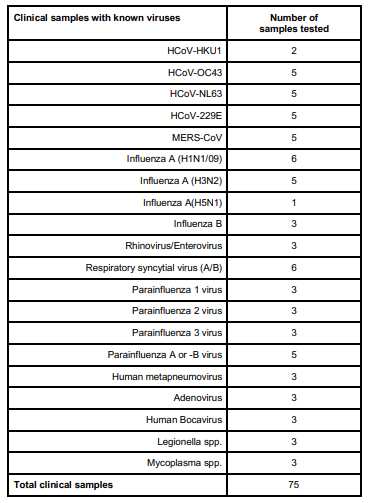
Affidabilità del campione
Quanto sono affidabili questi test? Ci sono due tipi di affidabilità. Una relativa al test stesso, una relativa al campione che il test deve considerare.
Affidabilità relativa al test stesso
Ci sono delle situazioni per cui il test può “sbagliare”, ovvero può dire che c’è il Coronavirus se invece non c’è, oppure può dire che NON c’è il Coronavirus anche se esso è presente nel campione. Questo tipo di errore esiste, è contemplato, tuttavia è piuttosto raro.
Affidabilità relativa al campione
Il test si può condurre su un campione non ben fatto per tanti motivi: per esempio nel campione prelevato con il tampone ci può essere troppo poco virus (scarsa “carica virale”), oppure il tampone è stato raccolto in un momento della storia clinica dell’individuo in cui esso non ha una grande quantità di virus.

Fonti:
- (1) https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance
- (2) https://www.who.int/docs/default-source/coronaviruse/protocol-v2-1.pdf?sfvrsn=a9ef618c_2
Altri articoli per chi vuole approfondire: